In one Doctor Who episode, the Doctor, an
alien creature and the main protagonist of the series, get inside a Dalek, an
arch enemy of the Doctor, to figure out the cause of its aberrant behavior.
Thanks to some advanced nanotechnology, the doctor and his companions were able
to get small enough to go inside the Dalek. While we might have to wait for a
while before our doctors could be miniaturized and injected inside our body to
get rid of, say, cancer cells or an arterial block, with the current advances
in biotechnology, we may be able to treat some difficult-to-treat diseases in a
similar fashion without going through the miniaturization process. Brain
cancer, for example, is one of the hardest tumors to treat, and every year,
about 10,000 patients are diagnosed with glioblastoma (GBM), the most common
primary brain cancer. In a recent paper, Juli Bagó and her
team showed that, in the near future, it might be possible to inject engineered
cells in the tumor region and have those cells destroy GBM and thus prolong
patients’ lives.
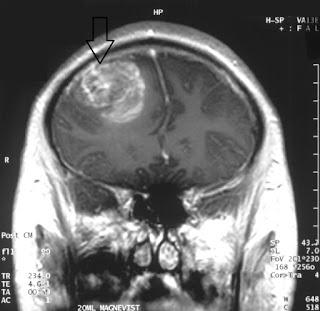 |
| MRI from a glioblastoma patient. Courtesy: Wikipedia |
To
achieve this exciting feat, the scientists needed cells with three different
properties: 1) the cells would avoid detection by immune cells, 2) they would
target tumors, and 3) they could be programmed to kill cancer cells.
Previously, it has been shown that neural stem cells (NSCs) engineered to
produce toxic agents can reduce GBM volumes by 70-90% and extend the survival
for mice. These cells, however, did not come from the host, so these cells do
not meet the first criterion, even though they meet the last two. In other
words, these cells would trigger an immune response from the host and will be
eliminated from the host unless used in conjunction with immunosuppressant
drugs. To circumvent the problem, the researchers took cells from connective
tissue like the skin from GBM patients and converted them to NSCs. Since the
cells came from the patients’ own bodies, these converted cells should not
trigger an immune response. Furthermore, this conversion process, called
transdifferentiation, is rather fast: the researchers managed to generate NSCs
within two days, so patients do not have to wait for a long time to get NSC
treatment.
Even
though these converted NSCs showed similar characteristics of actual NSCs, the
researchers had to confirm the second criterion: can these NSCs still target
GBM cells? To answer that question, the researchers took the converted NSCs and
monitored the interaction between NSCs and GBM. They found that not only these
converted cells migrated towards human GBM, but also penetrated GBM spheroids
in culture dishes that mimicked GBM tumors in the brain. These converted cells
thus fit the first two criteria we discussed before: they can be generated from
a patient’s own cells, and once generated, these cells can migrate to—and
penetrate—GBM.
Now, it
was time to tweak these cells so that once they contact GBM, they would kill
the cancer cells. The researchers engineered these converted NSCs to express a
toxin that would kill cancer cells: they produced a cell-death- or ‘apoptosis’-inducing
toxin called TRAIL (TNF-related apoptosis-inducing ligand)
in these cells. They then tested if these converted and engineered cells could
target and kill GBM cells. Compared to the cells that didn’t express TRAIL,
these cells reduced the viability of GBM spheroids in culture. They also
implanted GBM in mouse brains and monitored the tumor volume and survival rate
of these mice after the treatment of these engineered cells. They found that,
as expected, these engineered NSCs not only reduced the volume of the tumor
significantly but also prolonged these mice’s lives by two-fold (mice receiving
the engineered cells survived for 51 days compared to only 25 days in mice that
didn’t receive the treatment). The researchers also saw similar results in
culture when they engineered NSCs in a different way. NSCs engineered this way
can kill GBM by converting a non-toxic drug to a toxic compound, so the drug
needs to be present to turn on the “kill” feature in these cells. One exciting
feature of the cells engineered this way is that these cells attenuated the
regrowth of GBM in mice after removing the tumor with surgery, so NSC treatment
could lower the odds of cancer relapse after surgical removal of GBM.
While
these results are encouraging, it remains to be seen if the results from mice
can be replicated in human GBM patients. Furthermore, the efficacy and safety
of these engineered NSCs need to be rigorously tested before it is approved by
the FDA. In the meantime, cancer researchers might be able to engineer other
cell lines aimed for different types of cancer using a similar strategy. So for
now, the fancy future tech is already here in the form of stem cells; we may
just need to wait a bit longer to benefit from it.
